Research progress on Clostridium perfringens virulence factors and their mechanisms of action!
Time: May 9, 2022 Author: This site
Clostridium perfringens trivia
// First separation to:
In 1891, William H. Welch isolated air bubbles in infected blood vessels during the autopsy of a 38-year-old man. This characteristic would later link the occurrence of gastrax among British and French soldiers in World War I.
//The oldest trace:
In 1991, high-throughput sequencing and next-generation sequencing were used to identify the presence of Clostridium perfringens in the mummy digestive tract of Ötzi, the Neolithic "Tyrol Ice Man" who was more than 5,000 years old and was discovered in an Alpine glacier. .
//Full genome sequence published for the first time:
The first complete genome sequence of C. perfringens was released in 2002. This strain was isolated in 1939 and caused gas gangrene. It was also the first G+ anaerobic pathogen to be sequenced.

■ Clostridium perfringens is a normal flora in the intestines of animals and humans and is an opportunistic pathogen:
- Anaerobic (not strictly anaerobic), Gram-positive bacteria (G+);
- The bacterial body is fusiform, with blunt ends at both ends; it has no flagella and is immobile; it can form capsules and oval spores;
-Grows very quickly. In the optimal culture medium, the generation time is 8-12 minutes at 43°C and 12-17 minutes at 37°C.
■ Clostridium perfringens can produce a variety of virulence factors (including toxins and hydrolytic enzymes), allowing it to grow rapidly, tolerate aerobic environments, produce cytotoxic hydrogen sulfide gas, and cause toxicity to host tissues and intestines Infections, gas gangrene, enterotoxemia and other diseases.
- More than 20 virulence factors (23 species) have been identified. According to their role in intestinal infection, they can be divided into pore-forming toxins, membrane-destroying toxins, cytoskeleton-destroying toxins, and tight junction-destroying toxins. Mucus hydrolase, matrix lytic enzyme, collagenase. These toxins and enzymes can act alone or synergistically to destroy the normal physiological structure and function of host cells and tissues and harm animal health.
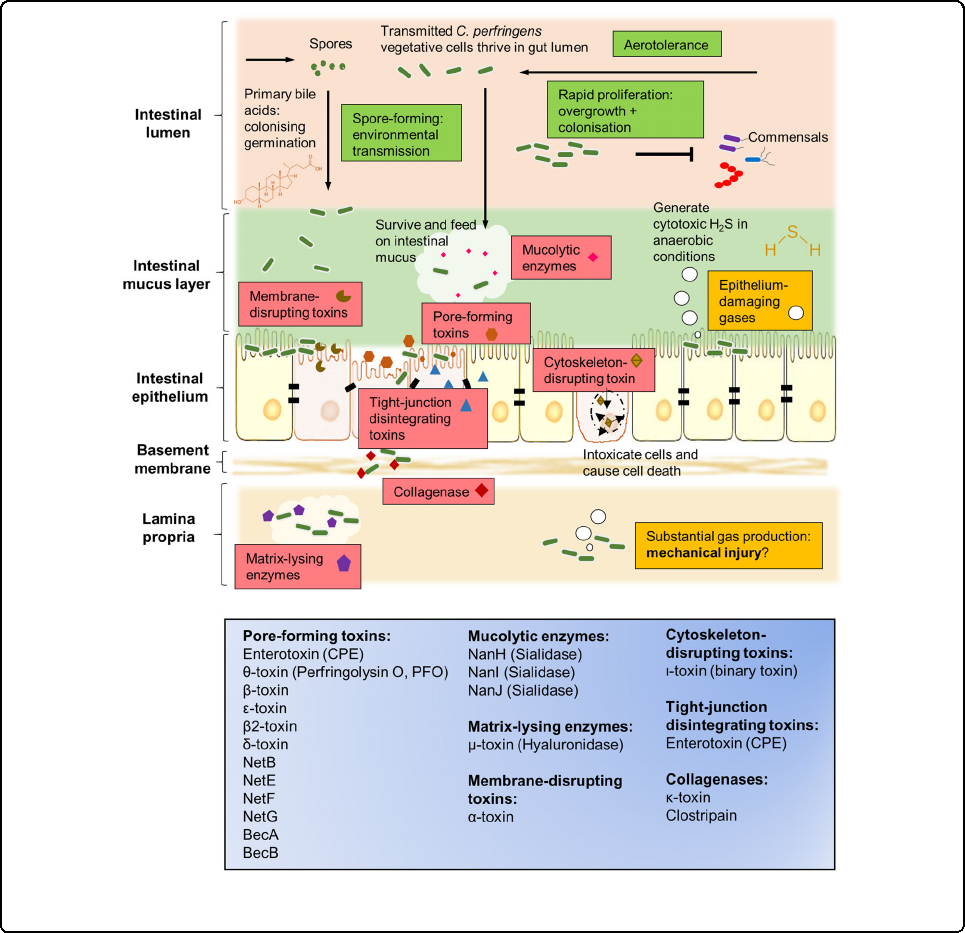
- Different strains produce different types of virulence factors, and no single strain of bacteria has been found to produce all virulence factors.
- Clostridium perfringens can be divided into 7 types from A to G based on the main types of toxins it produces (α, β, ε, ι, θ, CPE, NetB). (Note: "Minor" toxins do not mean that the importance or production level of these toxins is "minor", but that they do not belong to the toxin classification system)
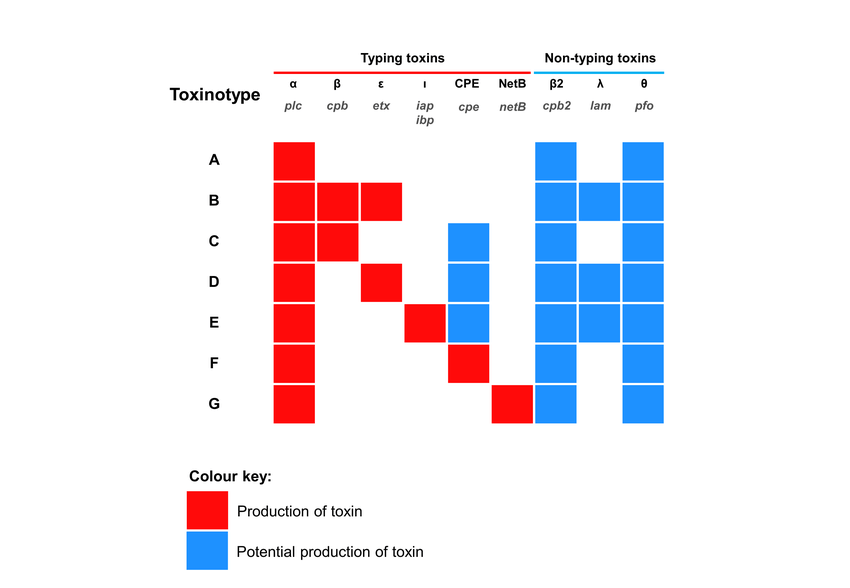
■ According to existing research reports on Clostridium perfringens in the breeding industry, the main toxins related to chicken intestinal diseases are α and NetB
α-toxin(CPA)
- Can be produced by all Clostridium perfringens bacteria and cause cell necrosis by hydrolyzing phospholipids in the cell membrane.
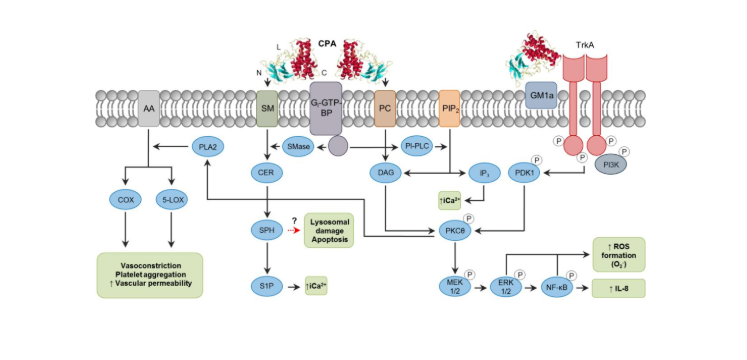
- CPA is a 370-amino-acid zinc metallophospholipase that binds to host cell membranes in the presence of calcium ions. Its structure includes: N-catalytic domain, C-membrane binding domain (with immune protection), central loop domain (containing ganglioside binding site);
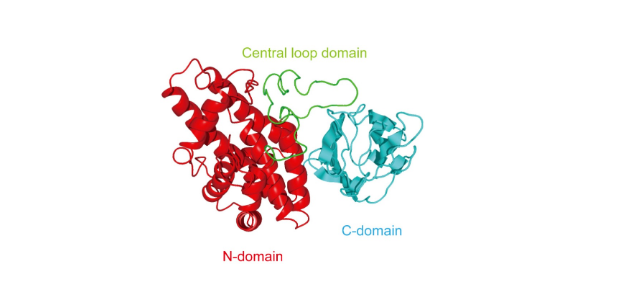
- The activity of CPA is affected by many factors, such as the ratio of phosphatidylcholine (lecithin, PC) to sphingomyelin (SM) in the cell membrane, local toxin concentration, etc.;
- CPA is an essential toxin that causes gas gangrene. Its effect is mainly achieved by affecting a variety of intracellular metabolic regulatory pathways:
① Directly hydrolyze PC and SM on the cell membrane;
② Acts with Gi-type GTP-binding protein (G-GTP-BP) in the plasma membrane to activate the activities of endogenous phospholipase (PI-PLC) and sphingomyelinase (SMase);
③The ganglioside binding site on the central ring region of CPA interacts with activated tropomyosin receptor kinase A (TrkA) on the cell membrane, phosphorylating PDK1 and PKCθ, activating MEK/ERK signaling and NF-κB, The formation of reactive oxygen species (ROS) causes cellular oxidative stress, activates the apoptosis mechanism, promotes IL-8 secretion, and promotes inflammation.
④ By destroying the integrity of GM1a lipid rafts on the cell membrane, blocking the differentiation and recruitment of peripheral blood neutrophils, and reducing the host's innate immunity.
⑤ Activates phospholipase A2 (PLA2), induces arachidonic acid (AA) catabolism, generates prostaglandins, leukotrienes, etc., causes vasoconstriction, platelet aggregation, reduces blood supply to infected tissues, and is beneficial to Clostridium perfringens of proliferation.
NetB toxin
- The encoding gene is located on the pathogenicity site NELoc1 of the conjugative plasmid. It consists of 293 amino acids and a β-pore-forming toxin of approximately 33kDa, which is 38% similar to β-toxin.
- There are relatively few studies on NetB toxins, but like other β-PFT toxins, it binds to the host cell membrane and assembles into oligomers to form transmembrane pores, destroying the phospholipid bilayer of target cells and causing sodium ions and chloride ions. , calcium ions, etc., causing cell lysis.
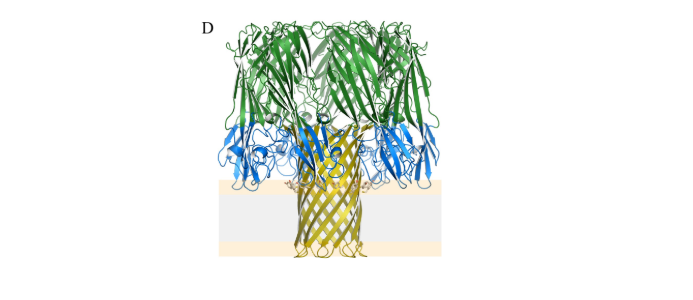
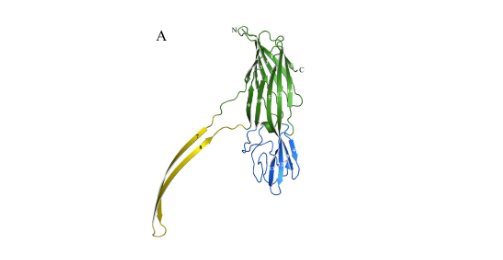
- NetB monomer is about 0.18nm and contains 3 main structural domains, as shown in Figure A: ①β-sandwich: green, related to monomer oligomerization; ②stem: yellow, hydrophobic transmembrane structure, inserted into the cell membrane; ③rim: blue Color, binds to the cell membrane, and participates in the penetration of toxins into the cell membrane; compared with other β-PFTs, it lacks 4 amino acids, and it is speculated that its receptors on the cell membrane are different from others.
- 7 NetB monomers aggregate in the cell membrane to form a hydrophilic pore with a diameter of 1.6–1.8 nm, as shown in Figure D: ①NetB pores preferentially transport cations; ②Cholesterol can promote the oligomerization of NetB monomers and play a role in pore formation plays an important role.