Research progress of proanthocyanidins in preventing and controlling secretory diarrhea (Part 2)
Time: May 19, 2022 Author: This site
This article has been published in "Chinese Animal Husbandry Magazine", Volume 57, Issue 04, 2021
Xu Jing1, Wang Xing1, Lei Lei1, Shuai Ke1,2, Yang Hanbo1,2*
(1. Slan Biology; 2. School of Veterinary Medicine, Sichuan Agricultural University)
Proanthocyanidins, also known as proanthocyanidins and condensed substances, are composed of flavan-3-ol and flavan-3,4-diol (catechin or epicatechin) as constituent units, which are polymerized by CC bonding. A class of flavonoid compounds commonly found in plants such as peanut shells, grape seeds, pine bark, sorghum bark, and dragon's blood croton, and has been proven to have antioxidant and anti-inflammatory functions [1-3]. In recent years, the anti-secretory diarrhea function of proanthocyanidins has been gradually discovered and confirmed . Secretory diarrhea is characterized by increased secretion of intestinal fluid caused by certain pathogenic bacteria (such as enterotoxin-producing Escherichia coli, Vibrio cholerae, etc.), viruses (such as rotavirus), inflammatory processes, drugs, genetics and other factors, and is accompanied by malabsorption. Diarrhea[4].

01 The structure of proanthocyanidins.
The basic building blocks of proanthocyanidins are flavan-3-ol and flavan-3,4-diol (mainly catechin or epicatechin). It is a typical flavonoid with a C6-C3-C6 structure. compound . Catechin or epicatechin is usually polymerized with C4-C8 or C4-C6 bonds. According to different degrees of polymerization, proanthocyanidins can be divided into oligomeric proanthocyanidins (degree of polymerization 2 to 5), polymeric proanthocyanidins (degree of polymerization >5, but still easily soluble in water), and high polymerization proanthocyanidins (high degree of polymerization and difficult to dissolve in water). )[1,3].
The content and degree of polymerization of proanthocyanidins from different plant sources vary greatly. Yamakoshi et al. [5] have shown that the proanthocyanidin content in grape seeds is 2% to 8% (based on dry weight of grape seeds), of which oligomeric proanthocyanidins can reach 25% . Liu Rui et al. [6] found that the content of proanthocyanidins in sorghum peel is 1% to 4%, but it is basically high polymerized proanthocyanidins. The properties of proanthocyanidins mainly depend on their degree of polymerization.

Proanthocyanidins contain a large amount of phenolic hydroxyl groups, which increase the degree of polymerization and enhance their astringent properties (binding with proteins to form water-insoluble tannic acid proteins). Proanthocyanidins with different degrees of polymerization have different degrees of difficulty in being absorbed by the intestines. The greater the degree of polymerization, the more difficult they are to be absorbed [7]. Research by Dens et al. [2] shows that proanthocyanidins with a polymerization degree of 2 to 3 are easily absorbed , 4 to 6 are slightly absorbed, and above 7 are basically not absorbed .
02 The main biological activities of proanthocyanidins
▶ Antioxidant activity: Proanthocyanidins are internationally recognized natural antioxidants with strong antioxidant properties. The antioxidant activity is 50 times that of vitamin E and 20 times that of vitamin C. Especially the antioxidant activity in the body, It is unmatched by other antioxidants [8]. The mechanism of action of proanthocyanidins is that they release H+ in the body, competitively combine with and react with free radicals. On the one hand, it can protect lipids from oxidation and block free radical chain reactions; on the other hand, half of the proanthocyanidins produced after the reaction can Quinone free radicals then generate polymers with catechin and pyrophenol structures through nucleophilic addition reactions, which still have strong antioxidant activity [9-10]. The antioxidant activity of proanthocyanidins is related to concentration, degree of polymerization, hydroxyl position, connection method, spatial configuration, solvent, etc. Its activity has a dose-effect relationship. When it exceeds a certain concentration range, its concentration increases and its activity decreases [8].

The activity generally increases as the degree of polymerization increases; methoxylation and glycosylation at the C3 position will reduce the activity; the activity of the C4-C8 connection is greater than the C4-C6 connection; after polymerization with epicatechin as the unit The activity is greater than the polymerization with catechin as unit; the activity is enhanced in the water phase and reduced in the oil phase [11].
Yin Mengyu[12] used ABTS, DPPH free radical scavenging method and pyrogallol autoxidation method to measure the antioxidant properties of proanthocyanidins in vitro, confirming that proanthocyanidins have significant in vitro antioxidant effects; selected the most representative and stable proanthocyanidins in vivo The specific 8-hydroxydeoxyguanosine DNA was used as the test object and compared with vitamin C + vitamin E tablets. The antioxidant effect of proanthocyanidins was better than that of the vitamin C + vitamin E group.

▶ Other biological activity research: In addition to antioxidants, domestic and foreign scholars have conducted a lot of research on the activities of proanthocyanidins, mainly including lowering blood lipids, anti-inflammation, anti-atherosclerosis, anti-cancer, regulating blood sugar, neurological diseases, and anti-diarrhea. and other aspects [13-17]. Among them , proanthocyanidins have the most outstanding application results in anti-diarrhea . In 2012, the U.S. Food and Drug Administration (FDA) approved Fulyzaq (trade name: Crofelemer) for the treatment of diarrhea symptoms in AIDS patients; Crofelemer is extracted from the red latex of Euphorbiaceae dragon blood croton and is an average molecular weight of approximately The product of random polymerization of multiple proanthocyanidin monomers of 2200 ku [18]
Crofelemer is the second botanical drug approved by the FDA and the first oral botanical drug ,indicating that its safety , effectiveness and other aspects have been recognized . It is of unprecedented significance for medicine to enter the European and American markets and to enter the Western medicine system as prescription drugs. Tang Qingsong et al. [19] have shown that adding 500 mg/kg and 1000 mg/kg condensed tannins to the diet can improve the growth performance of weaned piglets and alleviate diarrhea, and the diarrhea rates are reduced by 7.72% and 60.97% respectively.
03 The mechanism of proanthocyanidins against secretory diarrhea
Research on the mechanism of proanthocyanidins against secretory diarrhea mainly focuses on acting as an ADP-ribosylation inhibitor or chloride channel inhibitor to block certain links in the pathway of secretory diarrhea.
▶ ADP-ribosylation inhibition: Proanthocyanidins can inhibit the ADP-ribosylation transferase activity of cholera toxin or heat-sensitive enterotoxin A subunit, block the conversion of NAD into ADP-R, and inhibit the subsequent diarrhea effect, that is, proanthocyanidins Inhibits ADP-ribosylation. In other words, subunit A is equivalent to the "initiator" of enterotoxin-producing bacteria causing secretory diarrhea, and proanthocyanidins can "fail" at the beginning of its activation.
Noda Kimitoshi et al. [29] used the agmatine analysis method to show that proanthocyanidins with a degree of polymerization of 2 to 6 can inhibit the production of ADP-ribosylated agmatine catalyzed by cholera toxin to varying degrees. The characterization method is to measure [adenine-14C ] ADP-ribosylated agmatine radioactivity, while catechin monomer does not have this inhibitory effect, and proanthocyanidins from different sources such as apples, rhubarb, grape seeds, etc. all have this effect; the rabbit ileal ligation test further showed that, 50 μg of proanthocyanidins completely inhibited the accumulation of fluid in the ligated intestine (10 cm) caused by 1 μg of cholera toxin.
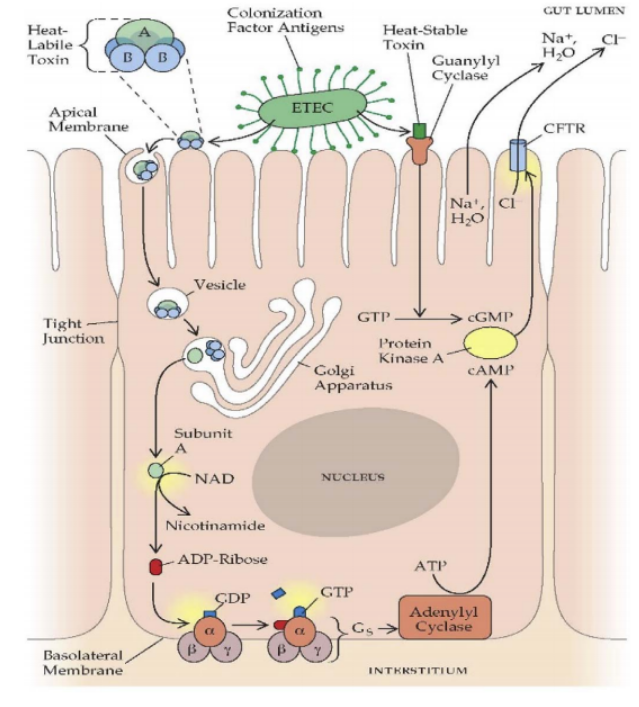
▶ Inhibition of chloride channels: The mechanism of antisecretory action of proanthocyanidins involves targeting and inhibiting 2 different intestinal chloride channels, namely the CFTR channel and CaCC, which are inhibitors of cAMP-stimulated chloride channels [30]. The chloride ion channel can be considered as the last gate that triggers the outflow of water and electrolytes, and proanthocyanidins "guard the last gate . " Tradtrantip et al. [31] have confirmed that Crofelemer inhibits the CFTR channel with a maximum inhibition rate of nearly 60% and an IC50 of 7 μmol/L. The voltage-dependent blocking effect produced at the extracellular site of CFTR stabilizes the closed state of the channel. And the maximum inhibition rate remained above 30% after 4 hours (reversal was less than 50%); further research found that Crofelemer passed a voltage-independent suppression machine.Crofelemer strongly inhibits CaCCTMEM16A, with a maximum inhibition rate > 90% and an IC50 of 6.5 μM; Crofelemer's dual inhibitory effect on two structurally unrelated intestinal Cl- channels may explain its intestinal anti-secretory activity. Poorvi [32] believes that Crofelemer is used to relieve non-infectious diarrhea symptoms in HIV-infected patients receiving antiretroviral therapy (ART), confirming that its mechanism is to inhibit chloride ions by blocking chloride ion channels in the gastrointestinal lumen. secreted, and has the advantages of good tolerability and safety.
Trials by Crutchley et al. [33] and Jessica et al. [34] have shown that Crofelemer can effectively reduce the amount of watery stools in patients with infectious diarrhea such as travelers' diarrhea and cholera , and significantly shorten the course of the disease . It is well tolerated when taken orally . In other words, Crofelemer can be used for both non-infectious and infectious secretory diarrhea. It should be noted that Crofelemer (SP-303) is a proanthocyanidin with a polymerization degree of 3 to 30 and an average molecular weight of 2,200 ku. Fisher et al. [35] compared SB-303 with SP-303, which came from the same source, was not separated and purified, the main component accounted for 67% of the mass of SP-303, and the average molecular weight was 3 000 ku, and the results showed that SB-303 also has anti-secretory properties. Although the diarrhea activity is slightly weaker than SP-303, the extraction process and cost are greatly reduced, which has high development value for areas that do not have high purity requirements (such as feed).

04 Prospects of proanthocyanidins against secretory diarrhea.
One of the important ways to improve breeding production efficiency is that animals do not get sick or have less disease. However, the prevention and control of infectious diarrhea in weaned piglets has always been a pain point and difficulty in pig breeding. Proanthocyanidins achieve anti-secretory diarrhea by inhibiting one or more links of hypersecretion caused by enterotoxin or other substances. It should be noted that the use of proanthocyanidins alone can control secretory diarrhea to a certain extent, but ensuring the normal structural integrity and function of the intestinal mucosa is also the basic condition for resisting secretory diarrhea.
Therefore , the combined use of proanthocyanidins and intestinal mucosal protective substances will be an important means to combat secretory diarrhea in the future .



